Description
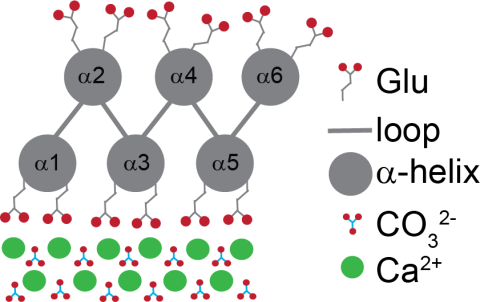
Biomineralization is the process by which organisms use biomolecules to produce hierarchically structured organic-inorganic composites. Using biology as inspiration, a protein construct (FD31) was previously designed to accelerate formation of nano-calcite with an unconventional {110} face. To understand the molecular interactions essential for protein aided calcite nucleation, solid-state nuclear magnetic resonance (ssNMR) spectroscopy was used in this work to characterize the FD31-calcite interface at the atomic level. Glutamic acid side chains designed to interact directly with calcium ions on the surface were found to have dynamics on the sub-millisecond timescale, indicating possible interactions between the protein and surface waters that were not included in the original model. Dipolar ssNMR recoupling techniques also showed that the protein backbone is ~2 Å closer to the surface than in the original docking model. Refined molecular simulations were done in the presence of explicit waters, which resulted in the protein backbone closer to the surface than in the original docking structure, providing better agreement with experiment and highlighting the important role played by water in FD31-calcite interactions. These studies provide the first experimental evidence to confirm that FD31 interactions with calcite are localized to the surface of the protein designed to serve as a template. However, these studies do indicate a more dynamic binding and closer binding mode between FD31 and the nucleated surface than originally proposed. In all, this enhanced molecular insight into the FD31-calcite interface has advanced our fundamental understanding of the atomic interactions at the organic-inorganic interface and will aid in the design of biological templates for the nucleation of inorganic crystals.