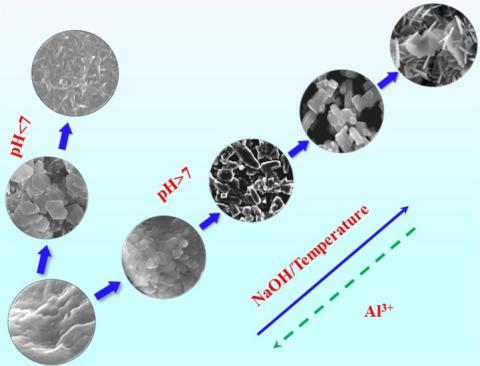
Gibbsite, bayerite, and boehmite are important aluminum (oxy)hydroxide minerals in nature and have been widely deployed in various industrial applications. They are also major components in caustic nuclear wastes stored at various U.S. locations. Knowledge of their crystallization and phase transformation processes contributes to understanding their occurrence and could help optimize waste treatment processes. While it has been reported that partial conversion of bayerite and gibbsite to boehmite occurs in basic solutions at elevated temperatures, systematic studies of factors affecting the phase transformation as well as the underlying reaction mechanisms are nonexistent, particularly in highly alkaline solutions. We explored the effects of sodium hydroxide concentrations (0.1–3 M), reaction temperatures (60–100 °C), and aluminum concentrations (0.1–1 M) on the crystallization and transformation of these aluminum (oxy)hydroxides. Detailed structural and morphological characterization by X-ray diffraction (XRD), scanning electron microscopy (SEM), and nuclear magnetic resonance (NMR) spectrometry revealed that these processes depend largely on the reaction temperature and the Al/OH– ratio. When 1 ≤ Al/OH– ≤ 2.5, the reactions favor formation of high-crystallinity precipitates, whereas at an Al/OH– ratio of ≥2.5 precipitation ceases unless the Al concentration is higher than 1 M. We identified pseudoboehmite, bayerite, and gibbsite as intermediate phases to bayerite, gibbsite and boehmite, respectively, all of which transform via dissolution–reprecipitation. Gibbsite transforms to boehmite in both acidic and weak caustic environments at temperatures above 80 °C. However, a “bar-shaped” gibbsite morphology dominates in highly caustic environments (3 M NaOH). The findings enable a robust basis for the selection of various solid phases by tuning the reaction conditions.
Publication - Journal Article Crystallization and Phase Transformations of Aluminum (Oxy)hydroxide Polymorphs in Caustic Aqueous Solution
Publication Image
Description
English
