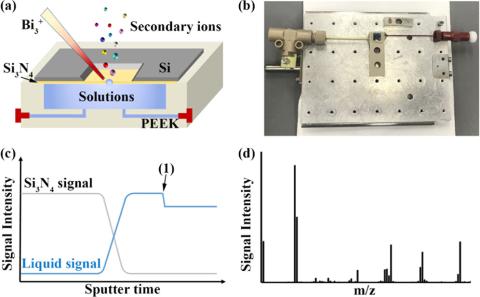
Understanding the structure and composition of aluminate complexes in extremely alkaline systems such as Bayer liquors has received enormous attention due to their fundamental and industrial importance. However, obtaining direct molecular information of the underlying ion–ion interactions using traditional approaches such as NMR spectroscopy or Raman spectroscopy is challenging due to the weakness of these interactions and/or their complex overlapping spectral signatures. Here, we exploit in situ liquid secondary-ion mass spectrometry (SIMS) as a new approach and show how it enables new insights. In contrast with traditional techniques, using SIMS we succeeded in acquiring information on dominant ion clusters in these alkaline systems. In Na+/K+ mixed alkaline aluminate solutions, we clearly observe preferential formation of Na+-anion clusters over K+-anion clusters. Evaluation of these clusters by density functional theory (DFT) calculations shows that these structures are stable and that their relative bond energies are consistent with their observed SIMS signal intensity differences. This demonstrates a key advantage of in situ liquid SIMS for overcoming ambiguities obscuring important information in these systems on constituent molecular clusters defined by relatively weak ion-pair competition and ion–solvent interactions.
Publication - Journal Article Molecular Examination of Ion-Pair Competition in Alkaline Aluminate Solutions Using In Situ Liquid SIMS
Publication Image
Description
English
