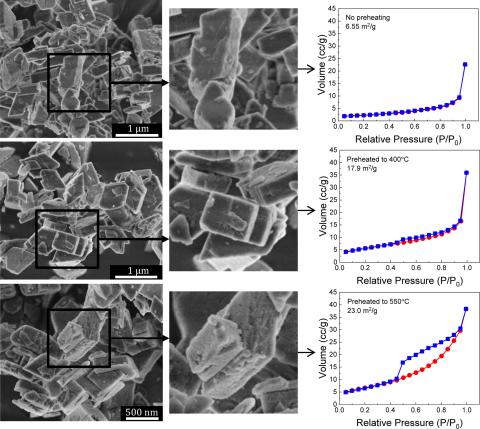
The stability of aluminum oxyhydroxide (AlOOH, boehmite) to radiolysis and dehydration to alumina (γ-Al2O3) under vacuum was investigated using TGA followed by detailed, structural analysis with Raman, powder X-Ray Diffraction (pXRD), high energy X-Ray Diffraction (heXRD), 27Al Magic Angle Spinning Nuclear Magnetic Resonance (27Al MAS NMR) spectroscopy, Scanning Electron Microscopy (SEM), and nitrogen adsorption. Slight structural deviations from annealing temperatures prior to phase transformation are evident using these techniques. Radiolytic characterizations by room temperature Electron Paramagnetic Resonance (EPR) spectroscopy showed that while boehmite preheated to 300 °C under vacuum remained crystalline boehmite as determined by bulk structural characterizations, this phase produced the largest amount of EPR-detectable defects. A more dramatic mass loss of 16% was achieved by preheating boehmite to 400 °C under vacuum that resulted in a phase similar to γ-Al2O3, but enriched with residual OH groups. Complete dehydration to γ-Al2O3 was accomplished by preheating to 550 °C, which showed no indication of residual OH groups. These results demonstrate that the residual OH groups contribute to the hydrogen gas evolved in the radiolysis of boehmite.
Publication - Journal Article Identification of Radiolytically-Active Thermal Transition Phases in Boehmite
Publication Image
Description
English
