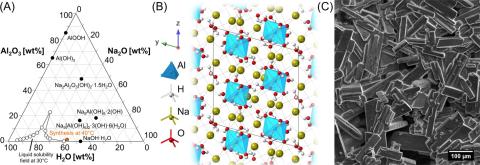
In highly alkaline “water-in-salt” Na2O/Al2O3/H2O solutions where the monomeric Al(OH)4– anion dominates, isolation of transitional species that seed crystallization of sodium aluminate salt hydrates has been challenging. For example, discrimination of dimeric [for example, Al2O(OH)62–] species via 27Al nuclear magnetic resonance (NMR) spectroscopy is limited via fast interconversion through hydrolysis and condensation reactions. Despite this, using magic-angle spinning NMR (27Al MAS NMR) at high magnetic field strengths (14.1 and 20.0 T) on crystals of nonasodium bis(hexahydroxyaluminate) trihydroxide hexahydrate [NSA, Na9[Al(OH)6]2·3(OH)·6(H2O)], we observed a 27Al resonance consistent with Na2Al2O(OH)6. This tetrahedral dimeric species was also identified in the mother liquor by Raman spectroscopy while remaining expectedly unresolved by high-field liquid-state 27Al NMR. Its substantial abundance in the mother liquor and as either an interstitial or an adsorbate on precipitated solids suggest that it plays a crucial role in NSA crystallization. Our detailed characterization, which includes single-pulse 27Al MAS NMR, nutation 27Al MAS NMR, and multiple quantum 27Al MAS NMR, provides new molecular insights into how aluminate transitions from tetrahedral in solution to octahedral in solid. This understanding could ultimately lead to better predictions of aluminum phase change behavior in caustic sodium hydroxide with wide-ranging technological and environmental implications.
Publication - Journal Article Intermediate Species in the Crystallization of Sodium Aluminate Hydroxy Hydrates
Publication Image
Description
English
