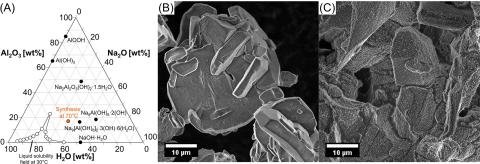
Crystallization of Al3+-bearing solid phases from highly alkaline Na2O:Al2O3:H2O solutions commonly necessitates an Al3+ coordination change from tetrahedral to octahedral, but intermediate coordination states are often difficult to isolate. Here, a similar Al3+ coordination change process is examined during the solid-state recrystallization of monosodium aluminate hydrate (MSA) to nonasodium bis(hexahydroxyaluminate) trihydroxide hexahydrate (NSA) at ambient temperature. While the MSA structure contains solely oxolated tetrahedral Al3+, the NSA structure is a molecular aluminate salt solely based upon monomeric octahedral Al3+. Spontaneous recrystallization of MSA and excess sodium hydroxide hydrate into NSA over 3 days of reaction time was clearly evident in X-ray diffractograms and in Raman spectra. In situ single-pulse 27Al magic angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy and 27Al multiple quantum (MQ) MAS NMR spectroscopy showed no evidence of intermediate aluminates, suggesting that transitional states, such as pentacoordinate Al3+, are short-lived and require spectroscopy with greater time resolution to detect. Such research is advancing upon a detailed mechanistic understanding of Al3+ coordination change mechanisms in these highly alkaline systems, with relevance to aluminum refining, corrosion sciences, and nuclear waste processing.
Publication - Journal Article Solid-State Recrystallization Pathways of Sodium Aluminate Hydroxy Hydrates
Publication Image
Description
English
