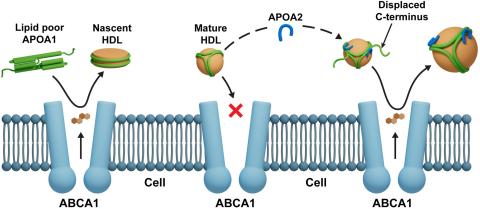
The ability of high-density lipoprotein (HDL) to promote cellular cholesterol efflux is a more robust predictor of cardiovascular disease protection than HDL-cholesterol levels in plasma. Previously, we found that lipidated HDL containing both apolipoprotein A-I (APOA1) and A-II (APOA2) promotes cholesterol efflux via the ATP-binding cassette transporter (ABCA1). In the current study, we directly added purified, lipid-free APOA2 to human plasma and found a dose-dependent increase in whole plasma cholesterol efflux capacity. APOA2 likewise increased the cholesterol efflux capacity of isolated HDL with the maximum effect occurring when equal masses of APOA1 and APOA2 coexisted on the particles. Follow-up experiments with reconstituted HDL corroborated that the presence of both APOA1 and APOA2 were necessary for the increased efflux. Using limited proteolysis and chemical cross-linking mass spectrometry, we found that APOA2 induced a conformational change in the N- and C-terminal helices of APOA1. Using reconstituted HDL with APOA1 deletion mutants, we further showed that APOA2 lost its ability to stimulate ABCA1 efflux to HDL if the C-terminal domain of APOA1 was absent, but retained this ability when the N-terminal domain was absent. Based on these findings, we propose a model in which APOA2 displaces the C-terminal helix of APOA1 from the HDL surface which can then interact with ABCA1-much like it does in lipid-poor APOA1. These findings suggest APOA2 may be a novel therapeutic target given this ability to open a large, high-capacity pool of HDL particles to enhance ABCA1-mediated cholesterol efflux.
Reference Citation
Sarkar, Snigdha et al. “APOA2 increases cholesterol efflux capacity to plasma HDL by displacing the C-terminus of resident APOA1.” Journal of lipid research vol. 65,12 (2024): 100686. doi:10.1016/j.jlr.2024.100686
Projects (2)
Predictive Phenomics Initiative (PPI) Project Data Catalog Collection The Predictive Phenomics Initiative (PPI) is an internal LDRD investment at Pacific Northwest National Laboratory focused on unraveling the mysteries of molecular function in complex biological systems. Explore PPI research...
Category
Datasets
18
The science objective of this project is to apply structural proteomics technologies to map the molecular interactome.
Category
Datasets
5