Aluminate salts precipitated from caustic alkaline solutions exhibit a correlation between the anionic speciation and the identity of the alkali cation in the precipitate, with the aluminate ions occurring either in monomeric (Al(OH)4–) or dimeric (Al2O(OH)62–) forms. The origin of this correlation...
Filter results
Tags
- (-) Proteomics (3)
- (-) Sodium (3)
- Omics (16)
- Viruses (10)
- Health (8)
- Soil Microbiology (8)
- Virology (8)
- Virus (8)
- Genomics (7)
- High Throughput Sequencing (7)
- Imaging (5)
- Mass Spectrometer (5)
- PerCon SFA (5)
- Sequencer System (5)
- Ions (4)
- Microbiome (4)
- Nanoparticles (4)
- sequencing (4)
- Spectroscopy (4)
- Electrical energy (3)
- Energy (3)
- Fungi (3)
- Mass Spectrometry (3)
- metabolomics (3)
- Microscopy (3)
- RNA Sequence Analysis (3)
- Synthetic Biology (3)
- Microarray (2)
- Polymer Materials (2)
- X-Ray Spectroscopy (2)
In highly alkaline “water-in-salt” Na2O/Al2O3/H2O solutions where the monomeric Al(OH)4– anion dominates, isolation of transitional species that seed crystallization of sodium aluminate salt hydrates has been challenging. For example, discrimination of dimeric [for example, Al2O(OH)62–] species via...
Crystallization of Al3+-bearing solid phases from highly alkaline Na2O:Al2O3:H2O solutions commonly necessitates an Al3+ coordination change from tetrahedral to octahedral, but intermediate coordination states are often difficult to isolate. Here, a similar Al3+ coordination change process is...
Last updated on 2024-03-03T02:26:52+00:00 by LN Anderson The Thermo Scientific™ Q Exactive™ Plus Mass Spectrometer benchtop LC-MS/MS system combines quadruple precursor ion selection with high-resolution, accurate-mass (HRAM) Orbitrap detection to deliver exceptional performance and versatility...
Last updated on 2024-03-03T02:26:52+00:00 by LN Anderson The Thermo Scientific™ Velos Pro™ Orbitrap Mass Spectrometer instrument data source combines advanced mass accuracy with an ultra-high resolution Orbitrap mass analyzer for increased sensitivity, enabling molecular weight determination for...
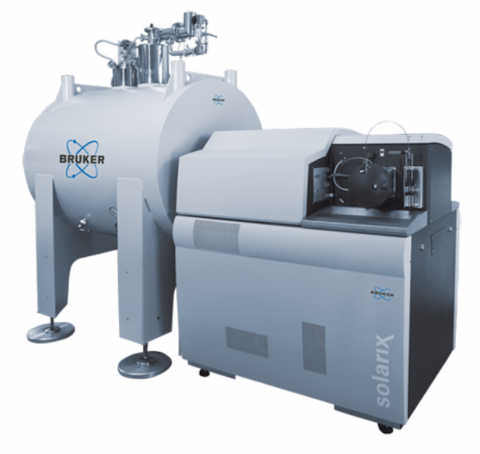
Bruker Daltonics SolariX Magnetic Resonance Mass Spectrometry (MRMS) instruments are available for different magnetic field strengths of 7T, 12T and 15T. The SolariX XR and 2xR instruments use magnetron control technology and a newly developed, high sensitivity, low noise preamplifier to exploit...